 Those of us who work with stallions are routinely asked to determine a stallion’s fertility prior to breeding mares or to investigate the cause of low fertility. Often this requires sending the stallion to a specialized veterinary clinic or a veterinary school. Advances have been made in stallion fertility evaluations through the use of computerized sperm motion analyzers (CASA) and flow cytometry.
Those of us who work with stallions are routinely asked to determine a stallion’s fertility prior to breeding mares or to investigate the cause of low fertility. Often this requires sending the stallion to a specialized veterinary clinic or a veterinary school. Advances have been made in stallion fertility evaluations through the use of computerized sperm motion analyzers (CASA) and flow cytometry.
Flow Cytometry
Flow cytometry allows simultaneous evaluation of several components of the sperm such as the plasma membrane, acrosome, mitochondria, etc. At the present time, most flow cytometers are at universities because of the expense and the need for a trained technician to operate them. Analysis is typically performed on fresh semen which requires the stallion be at the same facility as the flow cytometer.
With the advent of new staining procedures sperm can be stained on the farm and fixed in a solution so the stain does not change. Stained samples can then be sent to laboratories that have flow cytometry expertise for evaluation. This new technology opens the door for a greater number of samples from stallions to be evaluated and stallions can be located long distances from the laboratory. Recently in 2018, three studies validated the use of these novel stains for stallion semen evaluation.
Review of Recent Research
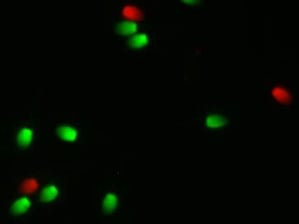
When Fernando Pena was on sabbatical leave from his university in Spain, he conducted a study with myself and Dr. Ball at the University of Kentucky. The aim of the study was to validate the use of a combination of fixable dyes to simultaneously assess the percentage of live/dead sperm and to determine the percentage of sperm with highly active mitochondria within the live sperm population. Three ejaculates from each of five stallions were collected and evaluated. For staining, cells were centrifuged and resuspended in 100ul of PBS to a concentration of 1-5 million sperm. Then 1 ul of Zombie Green and 1 ul of Mitotracker Deep Red were added to each sample and then incubated at room temperature for 30 min. After incubation, cells were washed by centrifugation and resuspended in PBS as unfixed samples or fixed in 2% paraformaldehyde then stored in the dark for 72 hours. They concluded that stained and fixed samples stored for 72 hours provided equivalent results for the percentage of live/dead sperm and active mitochondria compared to unfixed samples processed on the flow cytometer the day of collection.
A study conducted at Colorado State University (Trentin et al, 2018) also compared the flow cytometry results of unfixed versus fixed samples. The aim was to compare two flow cytometric methods for determining the percentage of live sperm and to optimize the incubation interval for staining of frozen/thawed semen. The non-fixed samples were incubated with the classic Syber -14/PI stain. A second aliquot was incubated with the fixable stain (Far Red Cell stain). Stained samples were collected at 10, 20, 30 and 40 minutes then fixed in paraformaldehyde for 20 minutes. After incubation, these samples were washed and resuspended in PBS and stored for 48 hours in the dark. The percentage of viable sperm for samples incubated for 20-40 min was similar to the non-fixed samples. This result indicates that when the sperm were incubated with the fixable stain for at least 20 min the results were equivalent to the non-fixed samples.
Teague et al (2018) at Texas A&M also performed studies to optimize the use of these fixable stains. They evaluated sperm concentration for staining, the concentration of the stain, incubation time of sperm with the stain, incubation temperature and diluents. Assay values were optimized when sperm were diluted to 2 million/ml with PBS/PVA followed by addition of 20 ul of stain and incubation for 10 minutes. They indicated that in a follow up study this procedure allowed the storage of fixed stained samples for 72 hours without affecting viability values compared to non-fixed samples.
These three studies are in good agreement and provide the information to construct a protocol for handling samples in the field. Select Breeders Services (SBS) has acquired a flow cytometer at the Maryland facility and have trained a scientist to operate the flow. Addition of this diagnostic technology to the main quality control laboratory at SBS will further enhance the ability to optimize cryopreservation protocols for clients of SBS and their affiliates. The use of these fixable stains will provide another tool to help us evaluate fertility in stallions and the response of sperm to being cooled or frozen.
References
Pena FJ, BA Ball and EL Squires 2018.A New method for Evaluating stallion Sperm Viability and Mitochrondrial Membrane Potential in Fixed Semen Samples. Cytometry Part B 94B :302 -311.
Trentin et.al 2018. Fixable Stain for Flow Cytometric Evaluation of Stallion Sperm Viability. Journal of Equine Veterinary Science 66:70
Teague et.al. 2018. Validation of a Fixable Stain for Assessing the Viability of Stallion Sperm. Journal of Equine Veterinary Science 66:42





