 Dr. Glenn Blodgett is the horse division manager at the 6666 Ranch, a member of the SBS Affiliate Laboratory Network and the current president of the American Quarter Horse Association. In recent years, Dr. Blodgett has implemented the use of chemical ejaculation with one of the stallions in his care. In this article, he will share with us in what situations chemical ejaculation might be useful, recent research performed using the procedure, how he uses it in his breeding program and the success rate of his attempts.
Dr. Glenn Blodgett is the horse division manager at the 6666 Ranch, a member of the SBS Affiliate Laboratory Network and the current president of the American Quarter Horse Association. In recent years, Dr. Blodgett has implemented the use of chemical ejaculation with one of the stallions in his care. In this article, he will share with us in what situations chemical ejaculation might be useful, recent research performed using the procedure, how he uses it in his breeding program and the success rate of his attempts.
Under certain circumstances and conditions it may be necessary to collect semen from a stallion when normal means of collection with an artificial vagina is not able to be utilized. Such as with stallions who suffer from severe laminitis, have recently undergone colic surgery, have hock and/or stifle injuries, age related issues, etc. Several compounds have been evaluated for inducing ejaculation in such stallions; imipramine alone or imipramine in combination with xylazine or detomidine. PGF2α has also been used but resulted in too many side effects.
Recent Research
It is important to note that although this technique is referred to as a Chemical Ejaculation there is no forceful expulsion of sperm from the ampullae and there is minimal contribution of fluid from the accessory sex glands. Therefore, this technique yields more of an emission of highly concentrated sperm per milliliter of fluid due to minimal seminal plasma provided by the accessory sex glands.
Several studies have been published by Dr. Sue McDonnell at the University of Pennsylvania. In one study (McDonnell and Odian 1994) they attempted semen collection from eight stallions on six occasions at four day intervals. The treatment utilized was imipramine alone or imipramine plus xylazine. They reported a success rate of 33% with success being slightly better when stallions were not sexually stimulated.
 In addition, Dr. McDonnell provided a review on chemical ejaculation in a 2001 report (McDonnell 2001). She reported that the success rate of inducing ejaculation ranged from 30-75%. The combination of 3mg/kg of imipramine given orally two hours prior to Xylazine (0.66mg/kg ) resulted in 44 ejaculations in 68 attempts or 65% of the time. The interval to ejaculation ranged from 1.2 to 14 minutes. The stallions either ejaculated at the beginning of sedation (1-4 minutes) or when coming out of sedation (14-15 minutes). With this combination of drugs the stallions produced a low volume, high concentration sample with greater sperm numbers then the same stallions collected prior with an artificial vagina (AV). Dr. McDonnell suggested that it may be worthwhile to do a dose titration of Xylazine for each stallion if one plans to collect semen by chemical means for an extended period of time.
In addition, Dr. McDonnell provided a review on chemical ejaculation in a 2001 report (McDonnell 2001). She reported that the success rate of inducing ejaculation ranged from 30-75%. The combination of 3mg/kg of imipramine given orally two hours prior to Xylazine (0.66mg/kg ) resulted in 44 ejaculations in 68 attempts or 65% of the time. The interval to ejaculation ranged from 1.2 to 14 minutes. The stallions either ejaculated at the beginning of sedation (1-4 minutes) or when coming out of sedation (14-15 minutes). With this combination of drugs the stallions produced a low volume, high concentration sample with greater sperm numbers then the same stallions collected prior with an artificial vagina (AV). Dr. McDonnell suggested that it may be worthwhile to do a dose titration of Xylazine for each stallion if one plans to collect semen by chemical means for an extended period of time.
As demonstrated by the report of Feary et.al 2005, chemical ejaculation can be used to collect semen from injured stallions and then frozen for subsequent use. In this study, a 20 year old stallion with priapism (unable to get an erection) was collected 5 times in 15 attempts over 39 days and the semen subsequently frozen.
Case Study Performed at the 6666 Ranch
In my particular case, I had a stallion at the 6666 Ranch that developed laminitis in both front feet and was over 20 years of age but still in good health otherwise. It became stressful and difficult for him to make the trip to the collection room on an every other day basis. Also, he is a stallion that requires considerable teasing and in some cases would require multiple jumps to collect semen from him in an AV. So I began to explore other options such as chemical ejaculation.
I had noticed that upon sedation with detomidine for procedures with his feet that he would ejaculate. Also, in consulting with colleagues who were performing insurance and prepurchase exams on various stallions in training, they conveyed a similar message. In order to measure the stallion’s testes, the veterinarians would sedate the stallion with detomidine and in many instances the stallion would ejaculate. This information gave me a basis for my decision to attempt to chemically ejaculate my stallion in order to manage him during the breeding season.
My stallion weighs 550 kg (approximately 1200 pounds). Every day during the breeding season he is given 25-50 mg imipramine tablets orally twice a day. Preferably, it is given 1-2 hours prior to the administration of 3 mg of detomidine. The detomidine, given only on breeding days, is drawn up in a tuberculin syringe and administered intravenously with a 20 gauge by 1 ½ inch needle.
A sterile plastic specimen cup with a lid is pre-warmed in the incubator to 37.5 degrees Celsius. Also, an insulated protective cone is pre-warmed to 37.5 degrees Celsius. A small amount (7-10 ml) of pre-warmed INRA 96 extender is placed in the specimen cup and the cup is then place in the insulated protective cone. The volume of extender used is an estimate of the volume of semen anticipated to be collected. This volume can be adjusted after you have obtained a pattern with your stallion. The cup is kept within the cone until the stallion is nearing ejaculation.
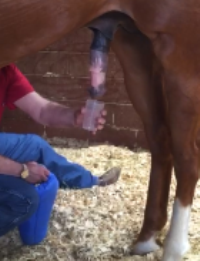
The collection occurs inside the stallions stall. Everyone is instructed to stay away and achieve as much privacy and as quiet an atmosphere as possible. In my particular case, the stallion does not mind me sitting on a plastic bucket at his side following administration of the detomidine. Ejaculation typically occurs within 2-5 minutes following administration of the detomidine. I monitor for no longer than 30 minutes post administration for ejaculation.
A characteristic movement occurs prior to the stallion approaching ejaculation. It resembles a twisting of the glans of the penis as can be observed in the video above. This twisting usually occurs two times. The observation of this characteristic movement will give you ample time to remove the specimen cup from the insulated cone and place beneath the glans in preparation for ejaculation. The ejaculate is of low volume and very high concentration. In my stallion’s case, it is usually 7-10 ml and over a billion cells per ml. Following collection, the cup with the sample is placed back in the protective cone and taken to the lab for evaluation and processing.
Conclusion
In summary, the above described procedure works for my stallion. However, it does not produce ejaculation 100% of the time. In some instances, we have been successful on three consecutive breeding or collection days on an every other day basis but more commonly we get him collected twice in a row followed by a day off. The end result is that we get him a minimum of at least twice a week. This procedure removes the stress this particular stallion would experience when walking to the breeding shed, tease for long periods of time and eventually mount the phantom. Now, he is able to remain in his stall while still producing offspring in a stress free environment.





